How it works.
How it works.
Healthcare Professionals.
Don’t lose sight of MM. Follow the M-protein through the patient’s deepest response.
EasyM™ is a CLIA-validated mass spectrometry-based blood test for minimal residual disease (MRD) monitoring in patients with Multiple Myeloma.
EasyM has been demonstrated to be an effective MRD assay for patients undergoing treatment for Multiple Myeloma in the clinic. The achievement of MRD negativity is strongly correlated with Progression Free Survival (PFS) and meets the criteria for PFS surrogacy.

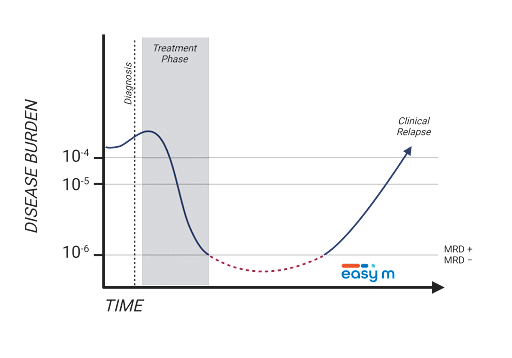
Continue to monitor M-Protein during the patient's deepest response to therapy with EasyM™
The achievement of MRD negativity is strongly correlated with Progression Free Survival (PFS) in MM.1,2 Although M-Protein is a well established biomarker for MM, conventional blood tests that measure M-protein (e.g. SPEP, IFE) lack the sensitivity needed to detect MRD.3 As such, highly sensitive next generation sequencing (NGS) and next generation flow cytometry (NGF) techniques are often used to assess MRD, but require painful and invasive bone marrow aspirations.4
EasyM™ is a non-invasive, high sensitivity mass spectrometry-based MRD blood test that allows direct and frequent monitoring of the patient’s M-Protein to enable physicians to track disease burden during the patient’s deepest responses to treatment.
A Simpler Way to Monitor MM.
For Clinical Collaborations
The Multiple Myeloma community benefits from exploration of MRD techniques using real-world clinical data. Contact us if you are interested in collaborations to explore the utility, comparative strengths and the applicability of EasyM™ MRD monitoring in your MM practice.
Physician Feedback
If you have feedback or suggestions about how we can improve EasyM™, or if you are looking for information about the Physician Feedback Program, please contact us for more information.
Keep in Touch
Follow us on LinkedIn and/or sign up below to receive news and updates on our progress to make EasyM into a widely available clinical test.
CLIA Certified.
EasyM has been approved as a Laboratory Developed Test (LDT) by CLIA/COLA in all US states except CA, NY, RI, and PA. We are seeking further certification in the remaining states, by the US FDA and by Health Canada.
Upcoming Conferences.
2024 conference calendar coming soon.
References
- Landgren, O. et al. Bone Marrow Transplant. (2016) 51:1565– 8.
- Kaddoura, M. et al. Am J Hematol. 2022; 97(3): 267- 273
- Murray, D.L. et al. Blood Cancer J. 2021;11(24)
- Kumar, S. et al. Lancet Oncol. 2016;17(8):e328-e346
- Liyasova M, et al. Clin Cancer Res. 2021; 27(18):5028-5037
- Slade M, et al. Blood 2022; 140 (Supplement 1): 4376–4377.



